Ethylene Oxide Production Co2
Mixed- metal oxides were identified and tested in a micro-reactor for ethylene oxide yield. During this time the concentration of carbon dioxide in the eflluent gas remained essentially constant at 0006 volume percent 60 ppm.
We systematically screened binary catalysts for synthesis of dimethyl carbonate DMC from CO 2 CH 3 OH and ethylene oxide EO in the presence of H 2 O by two-step transesterification process and found the commercial quaternary ammonium salts K 2 CO 3 pyridinium salts K 2 CO 3 and KIK 2 CO 3 were effective binary catalysts for two-step synthesis of DMC with no need for separation of ethylene.

Ethylene oxide production co2. Production and importance of ethylene oxide have steadily grown. The specifications for the system were to obtain approximately 10 conversion of ethylene and a 30. Ethylene oxide is an important feedstock for the chemical industry and is used to make many useful products such as polyurethanes polyols glycols nitriles alcoholamines and ethers.
Ethylene chlorohydrin was produced in either the same or a separate unit and was pumped over to the ethylene oxide production sector. In the manufacture of ethylene carbonate EC from ethylene oxide EO and carbon dioxide CO 2 wherein the EO and CO 2 are obtained as the purified products of the reaction of ethylene and oxygen over a silver catalyst ie via direct oxidation ethylene oxide DOEO an improved integrated process is obtained by absorbing the DOEO reactor effluent in ethylene carbonate desorbing stripping the. The reaction is highly exothermic and the heat removed can be used to generate steam.
These ethylene oxide. Together with ethylene oxide CO2 H2O and heat is generated as well. Low solubility hampers ethylenes interaction with the cells anode and reduces the efficiency of the oxidation process.
The Ethylene Oxide Derivatives Producers Associations EO GLYCOLS represent all major EU manufacturers of ethylene oxide. The direct use of ethylene oxide accounts for only 005 2004 data of its global production. Currently ethylene oxide is produced by direct oxidation of ethylene with air or oxygen.
1 Ethylene Oxide production plant Steps of oxygen-based ethylene oxide processing Feed of ethylene and oxygen into the reactor and oxidation of ethyl-ene by passing through the reactor bundles of tubes packed with the catalyst material at 200 to 300 C. Ethylene oxide is a chemical intermediate required for the manufacture of many important products and downstream marketsThe major use of ethylene oxide in Europe is as a chemical intermediate in the manufacture of ethylene oxide derivatives such as surfactants ethanolamines glycol ethers polyether polyols and other ethylene oxide derivatives. Ethylene oxide is used as a sterilizing agent disinfecting agent and fumigant as a mixture with carbon dioxide 8580 of ethylene oxide nitrogen or dichlorodifluoromethane 12 ethylene oxide.
The second reaction is the combustion of ethylene to carbon dioxide. Disclosed is a method for recovering carbon dioxide from an ethylene oxide production process and using the recovered carbon dioxide as a carbon source for methanol synthesis. CO2EXIDE CO 2-based electrosynthesis of ethylene oxide.
The production of ethylene chlorohydrin resulted in the formation of two main organochlorine by-products 12-dichloroethane and bis2-chloroethylether see IARC 1999a. System has been demonstrated that eliminates CO2 formation while producing ethylene oxide at 90 Ethylene selectivity at near-ambient temperatures. Reaction with calcium oxide.
Emissions of CO2 from ethylene oxide production may be estimated using emission factors based on activity data for ethylene oxide production and activity data for process configuration and catalyst selectivity. The main reaction is the formation of ethylene oxide from ethylene with approximately 81 selectivity towards this reaction. Two reactions for ethylene oxide production were considered for the reactor system which can be seen in Table 1.
Saturation of the bed with carbon dioxide was reached after. The tables of contents are generated automatically and are based on the data records of the individual contributions available in the ind. Boosting the power of the cell might improve the rate of ethylene oxide production but it also creates a second challenge.
It can overoxidize ethylene generating unwanted CO 2. Separate CO2 emission factors are provided in Table 320 for the CO2 emissions from the air process and for the CO2 emissions from the. One of the central steps is the development of a new type of electrolyser that enables a simultaneous reaction on both.
Additionally ethylene oxide can be formed through the reaction between ethylene and CO 2 forming CO as a byproduct which is a valuable raw material for manufacture of methanol and for other FT. The mixture is passed over a silver oxide catalyst supported on a porous carrier at 200-300oC and 10-30 bar. In 1931 LEFORT 9 discovered the direct catalytic oxidation of ethylene 74-85-1 which gradually superseded the chlorohydrin process.
The mixed-metal oxides were shown to produce ethylene oxide under moderate conditions. A conventional hydrocarbon feedstock that is of particular interest for CO2utilization is ethylene which is used to make ethylene oxide. Comparison of the single-pass yields of the CO2.
Annual worldwideproductioncapacityisca15 106 t. By judicious choice of the catalyst methyltriox- Available online 12 February 2009 orhenium oxidant H2 O2 and reaction medium methanolwater a homogeneous liquid phase catalytic Keywords. Table of contents conference proceedings.
More specifically carbon dioxide recovered from an ethylene oxide production process is used to produce a syngas stream. The goal of the project CO2EXIDE is the establishment of an electrochemical energy efficient and near-to CO 2-neutral process for the production of the bulk chemical ethylene from CO 2 water and renewable energy. The syngas stream is then used to produce methanol.
A process for the utilization of CO2 for the conversion of ethylene to ethylene oxide has been studied for the greenhouse gas life cycle analysis and compared to the commercial production of ethylene oxide which uses oxygen from an air-separation unit. Ethylene compressed oxygen and recycle gas are mixed and fed to a multi-tubular catalytic reactor.

Chloride Mediated Selective Electrosynthesis Of Ethylene And Propylene Oxides At High Current Density Science
Http Www Diquima Upm Es Old Diquima Docencia Tqindustrial Docs Ox Etileno Pdf
Petrochemical Chemical Industry Mogas

Optimal Design Of Gas Expanded Liquid Ethylene Oxide Production With Zero Carbon Dioxide Byproduct Industrial Engineering Chemistry Research X Mol
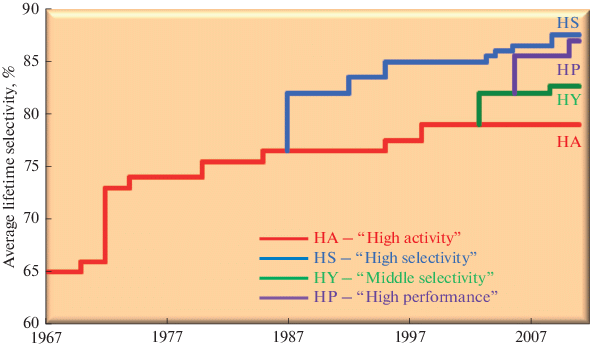
Prospects For The Development Of Ethylene Oxide Production Catalysts And Processes Review Springerlink

World Production Of Ethylene Oxide 2004 Download Table
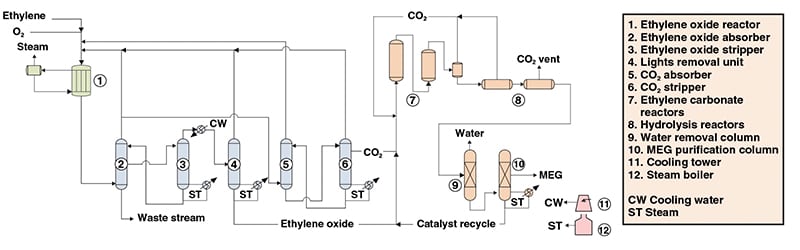
Ethylene Glycol Production Chemical Engineering
Https Www Ugr Es Tep028 Pqi Descargas Industria 20quimica 20organica Tema 5 Oxido Etileno A10 117 Pdf

Process Flow Diagram Of The Cebc Process Table 3 Lists The Simulation Download Scientific Diagram
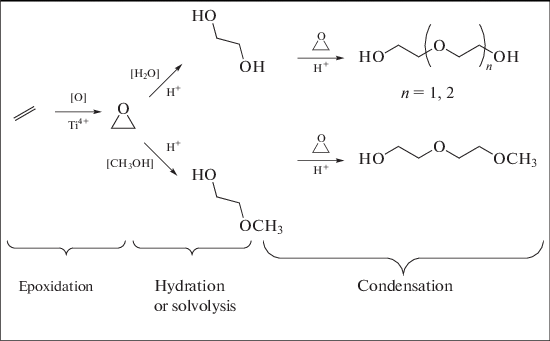
Prospects For The Development Of Ethylene Oxide Production Catalysts And Processes Review Springerlink

A Pfd For Ethylene Oxide Plant 4 Download Scientific Diagram
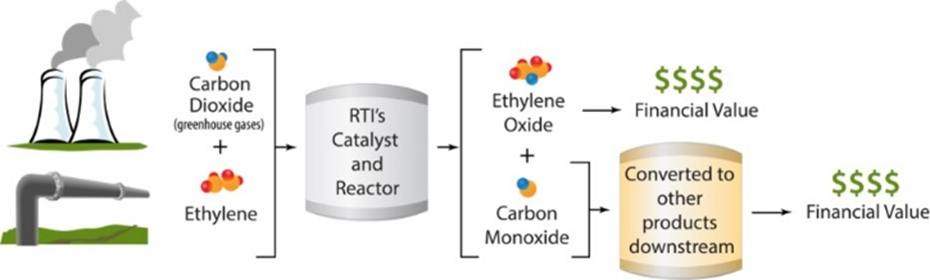
Novel Catalysts Process Technology For Utilization Of Co2 For Ethylene Oxide And Propylene Oxide Netl Doe Gov

Production Of Ethylene Oxide By Suraj Kansara
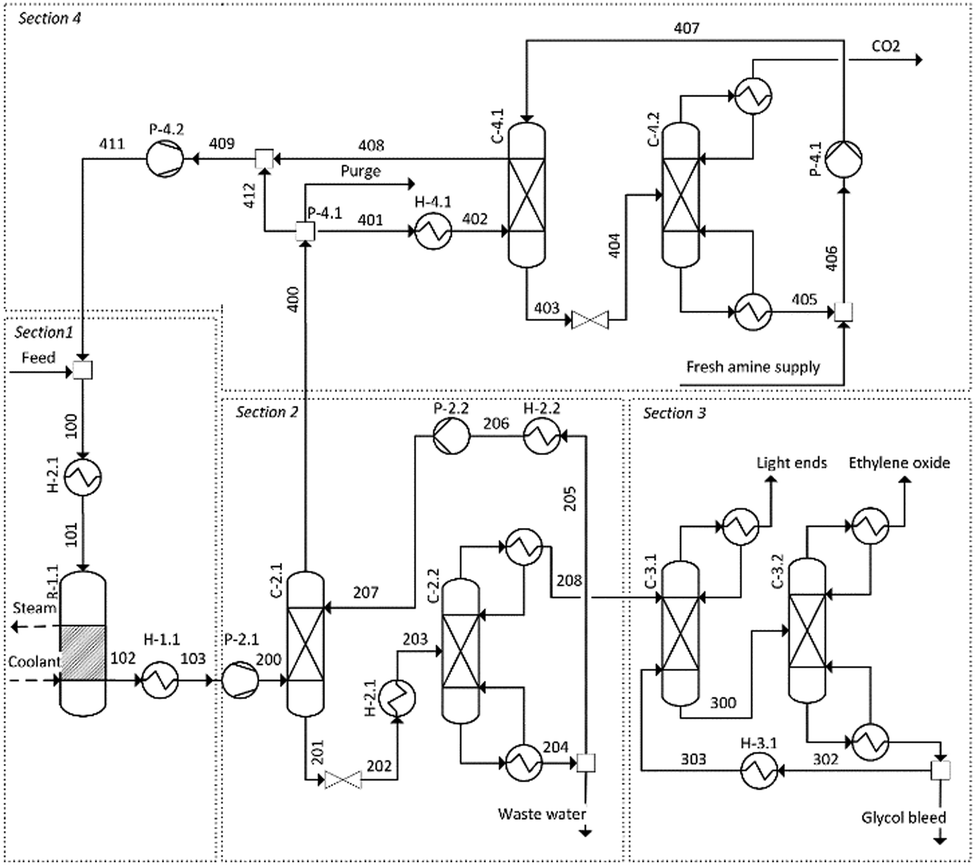
Economically Viable Co 2 Electroreduction Embedded Within Ethylene Oxide Manufacturing Energy Environmental Science Rsc Publishing Doi 10 1039 D0ee03310c

A Simulation Model Of A Reactor For Ethylene Oxide Production Semantic Scholar

Design And Optimize Ethylene Oxide Production Plant

Ethylene Oxide An Overview Sciencedirect Topics
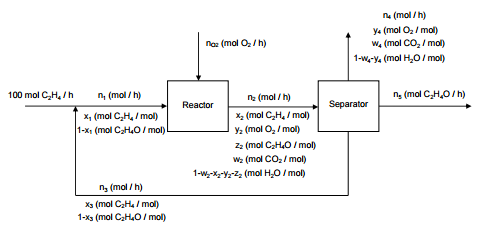
Ethylene Oxide Is Produced By The Catalytic Oxidation Chegg Com

Chloride Mediated Selective Electrosynthesis Of Ethylene And Propylene Oxides At High Current Density Science
0 Response to "Ethylene Oxide Production Co2"
Posting Komentar